When people hear “GMP warehouse,” they often imagine paperwork, inspections, and endless rules. In reality, GMP warehousing is what quietly protects product quality long after pharmaceutical manufacturing ends and before it ever reaches a customer. From temperature-sensitive medicines to supplements, cosmetics, and medical devices, the way pharmaceutical warehouses manage storage conditions can determine whether products remain safe, effective, and legally sellable under GMP regulations.
A GMP warehouse isn’t just a place to store goods – it’s a controlled system where infrastructure, people, and processes work together to ensure GMP compliance and long-term regulatory compliance. As ecommerce brands expand into regulated markets and global supply chain complexity increases, understanding good manufacturing practice in warehouse operations has become essential, not optional. This guide explains the meaning, GMP requirements, and practical realities of operating (or selecting) compliant pharmaceutical warehouses.
What does “GMP warehouse” mean?
A GMP (Good Manufacturing Practices) warehouse is a storage facility designed, operated, and controlled under good manufacturing practice rules to protect product quality, safety, and efficacy from receipt through the distribution process. Unlike conventional facilities that prioritise speed alone, pharmaceutical warehouses embed validated processes, temperature control, monitoring equipment, and proper documentation into daily warehouse operations to meet GMP standards and support reliable warehouse operations.
Originally developed for pharmaceuticals under key GMP regulations such as FDA 21 CFR Parts 210–211 and EU GMP guidelines, these principles now apply to supplements, cosmetics, medical devices, and other regulated pharmaceutical products. Any product vulnerable to contamination, temperature fluctuation, or handling errors requires storage aligned with GMP regulations.
Understanding the GMP warehouse comes down to two connected layers:
- Physical infrastructure: validated storage areas, cleanable surfaces, segregation zones, security measures, and calibrated monitoring equipment
- Quality systems: standard operating procedures, training, proper documentation, audits, quality control, and CAPA
A vaccine stored at 2–8°C depends on validated temperature control and staff trained to ensure compliance. A light-sensitive cosmetic serum requires proper packaging, proper labeling, and warehouse staff who understand quality standards.
GMP warehouse vs. standard warehouse
The difference between a GMP warehouse and a standard warehouse lies in priorities. Conventional facilities optimise for speed and cost. Pharmaceutical warehouses prioritise GMP compliance, traceability, and regulatory compliance, even if it reduces efficiency across ecommerce supply chain management workflows.
Standard warehouses track SKUs and quantities. GMP-compliant pharmaceutical warehouses track batches, expiry dates, and proper labeling, while enforcing security measures and controlled storage areas.
For example, protein powder requires batch traceability and recall readiness. Clothing does not. This distinction becomes critical when ecommerce brands enter regulated markets and must maintain compliance with GMP regulations.
GMP warehouses operate differently across several key dimensions:
- Environment: Pharmaceutical warehouses maintain controlled temperature and humidity ranges validated through temperature mapping exercises, while standard facilities typically offer only ambient storage without environmental monitoring
- Records: GMP facilities track every batch and lot number with expiry dates and maintain comprehensive records of where each unit has been stored, whereas standard warehouses count SKUs without batch-level visibility
- Regulatory oversight: GMP warehouses face inspections from bodies like the FDA, MHRA, or national competent authorities, while standard facilities answer primarily to occupational health and safety regulators
For example: storing protein powder requires batch traceability, temperature monitoring, and recall readiness. Storing clothing does not. This distinction often becomes clear when ecommerce brands expand into regulated markets like the EU, UK, or US – where GMP-compliant 3PLs replace conventional fulfillment centers.
Many ecommerce brands in beauty, wellness, and nutraceuticals discover this distinction when expanding into regulated markets like the US, EU, or UK. Selling a collagen supplement through Amazon or direct-to-consumer channels requires compliance with GMP regulations, which often means partnering with a GMP-compliant 3PL rather than using conventional fulfillment centers.
Core GMP warehouse requirements
While regulations differ globally, GMP standards consistently require six pillars that govern warehouse operations and ensure quality control across pharmaceutical warehouses – particularly for brands managing complex distribution management models.
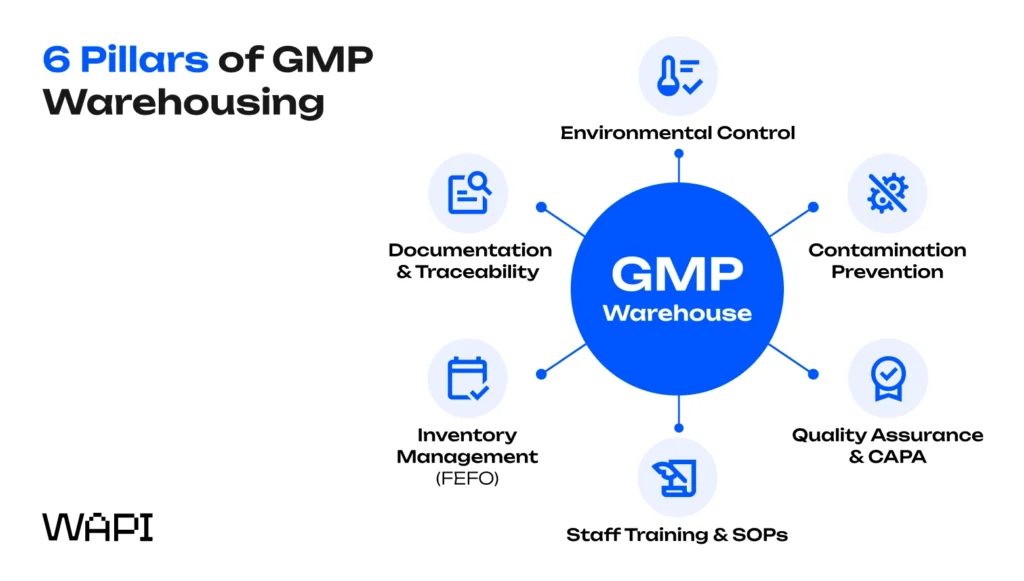
Each pillar is essential – weakness in one undermines the entire quality system. Let’s look at each one in more detail.
1. Documentation, traceability, and batch records
In GMP warehousing, the saying “if it isn’t written down, it didn’t happen” is more than a cliché – it’s a regulatory reality. Documentation is the proof that products were handled correctly at every stage of storage and distribution.
What must be documented in a GMP warehouse?
Rather than long narratives, GMP relies on structured records. Typical documentation includes:
- Goods receipt logs (supplier, batch, quantity, condition)
- Batch and lot location histories
- Continuous temperature and humidity logs
- Deviation and incident reports
- Cleaning and sanitation records
- Distribution and dispatch records
Together, these records create a complete, auditable history for every batch.
Why batch traceability matters?
When batch traceability is implemented correctly, it becomes critical during recalls. Imagine a blood pressure medication batch (ABC123) is recalled across the EU due to an issue identified at the manufacturing stage. A GMP warehouse can immediately determine whether that batch is still in stock, pinpoint its exact storage location, and identify every customer who received it. This level of precision prevents unnecessary broad recalls, limits financial and reputational damage, and most importantly, protects patients by ensuring affected products are removed quickly and accurately.
Regulators don’t expect warehouses to be flawless, but they do expect traceability. Being able to demonstrate what happened, when it happened, and how it was addressed shows control over the quality system. To support this, modern GMP warehouses rely on validated WMS or ERP platforms with audit trails and electronic signatures aligned with requirements such as 21 CFR Part 11 in the US or EU Annex 11. Every inventory movement – receiving, storage, picking, or dispatch – is permanently recorded, attributable to a specific user, and tamper-evident, ensuring that documentation presented during inspections accurately reflects real warehouse activity.
2. Environmental control and continuous monitoring
Temperature controlled warehousing is central to GMP standards. Storage ranges vary by product, and pharmaceutical warehouses must prove conditions were maintained continuously, not “most of the time.”
Typical GMP storage conditions
| Product type | Temperature range |
|---|---|
| Room-temperature medicines | 15–25°C |
| Cold chain products | 2–8°C |
| Frozen biologics | –20°C to –70°C |
| Hygroscopic products | Humidity <60% RH |
Achieving this requires more than air conditioning. Qualified HVAC systems, controlled airflow, insulated loading zones, and light protection all play a role.
Monitoring & alarms (how control is proven?)
Environmental control in a GMP warehouse is only meaningful if it can be continuously verified. To achieve this, facilities rely on calibrated sensors positioned at validated locations throughout the storage area, combined with uninterrupted data capture that records temperature and humidity in real time. Automated alarm systems are configured to alert personnel immediately when conditions move outside approved limits, triggering formal investigations and documented corrective actions.
For example, if the temperature exceeds 25°C for longer than 30 minutes, the system generates an alert, prompting staff to assess product impact, take corrective measures, and record the event in a deviation report to demonstrate ongoing control and compliance.
Temperature mapping
Before regulated products are stored, warehouses undergo temperature mapping studies. Dozens of data loggers are placed across the facility during summer and winter to identify hot and cold spots.
EU GDP Guidelines explicitly require this qualification step.
3. Contamination prevention, hygiene, and facility design
GMP warehouse design is about controlling risk through layout, materials, and movement. The goal is to prevent cross-contamination, mix-ups, and pest ingress before they happen. Effective GMP facilities typically include:
- Separate zones for quarantine, released, and rejected goods
- Smooth, cleanable floors and walls
- Controlled personnel and material flows
- Clearly marked storage areas and access points
Physical separation reinforces system-based controls in the WMS.
Hygiene & pest control essentials
GMP hygiene goes beyond surface-level cleanliness. It requires documented, verifiable controls that actively prevent contamination and demonstrate ongoing oversight.
- Documented cleaning schedules: Define what is cleaned, how often, with which approved agents, and by whom. Records prove that cleaning is performed consistently and according to validated procedures.
- Mapped pest control stations: Traps and monitoring points are placed at strategic locations and documented on facility maps to ensure complete coverage and traceability.
- Regular contractor inspections: Licensed pest control providers conduct routine inspections to detect early signs of infestation and verify that preventive measures remain effective.
- QA review of pest reports: Quality teams formally review inspection findings, assess trends, and ensure corrective actions are implemented and documented where needed.
Effective GMP facilities align physical design with operational controls, ensuring compatibility with broader on-demand warehousing and scalable fulfillment models.
Even minor pest evidence, such as a single insect finding, can trigger serious inspection findings, which is why proactive hygiene and pest control are treated as core GMP requirements, not optional measures.
Common pitfall: Shared tools or forklifts moving between GMP and non-GMP areas without cleaning validation.
4. Inventory management, FEFO, and status segregation
Unlike general logistics, GMP warehouses operate on FEFO (First Expired, First Out), ensuring products with the shortest remaining shelf life are distributed first. This approach supports strong stock control and minimizes the scope of recalls by preventing outdated batches from reaching customers.
FEFO vs FIFO
| FIFO | FEFO |
|---|---|
| Ships by arrival date | Ships by expiry date |
| Common in retail & general logistics | Mandatory in GMP environments |
| Higher expiry risk | Minimizes waste & recall exposure |
In GMP warehouses, the Warehouse Management System enforces FEFO logic automatically. Pickers are guided to the correct batch, while later-expiring stock is system-blocked until earlier batches are depleted, eliminating manual decision-making and reducing human error.
Status-based inventory control
Each batch is assigned a defined system status that determines whether it can move through the fulfillment process:
- Quarantine: Received but awaiting quality approval
- Released: Approved for distribution or use
- Rejected: Failed quality requirements and blocked from use
- Returned: Subject to investigation before disposition
- On hold: Temporarily restricted due to quality or regulatory review
Physical segregation – using color-coded labels, dedicated zones, cages, or locked areas – mirrors system status to prevent accidental picking or mix-ups.
Together, FEFO logic and strict status segregation allow GMP warehouses to execute targeted recalls within hours rather than days, limiting financial impact while maintaining full regulatory control.
5. Warehouse Staff Training and SOP adherence
GMP compliance depends on people just as much as systems. Even the most advanced warehouse infrastructure can fail if staff do not fully understand how regulated products must be handled. For this reason, people are central to gmp compliance. All staff must follow standard operating procedures, wear appropriate protective clothing, and understand how their actions impact quality control.
Training is role-specific and refreshed regularly, reinforcing continuous improvement and inspection readiness across pharmaceutical warehouses – especially important for teams supporting complex fulfillment models such as fulfilled by TikTok or high-volume D2C operations.
Training in a GMP warehouse is never one-size-fits-all. While core GMP and GDP principles apply to everyone, training must be role-specific, reflecting the risks and decisions associated with each position. A picker verifying batch numbers faces different risks than a forklift operator managing segregated zones, and both differ significantly from the responsibilities of quality assurance personnel reviewing deviations or approving stock release.
GMP training typically covers:
- GMP and GDP fundamentals, explaining regulatory expectations and product risk
- Hygiene and gowning requirements, ensuring personal behavior does not compromise product quality
- Batch identification and verification, preventing mix-ups and traceability gaps
- Handling of temperature-sensitive products, including excursion response
- Documentation rules, with emphasis on accuracy, timing, and accountability
- Incident escalation procedures, defining when and how issues must be reported
Beyond formal training sessions, regulators expect staff to demonstrate real understanding during inspections. Warehouse personnel are often interviewed and asked to explain why they perform tasks in a certain way, not just how. In practice, clear comprehension matters more than memorized procedures.
Training does not stop after onboarding. Refresher training is typically conducted at least once per year and fully documented, including training content, dates, and trainer identification. In higher-risk environments, competency checks – such as observed task performance or short assessments – are used to confirm that staff can consistently apply SOPs in day-to-day operations.
6. Quality assurance, GMP Regulations and CAPA
Quality assurance provides the governance layer that holds a GMP warehouse together. QA systems often operate alongside broader compliance frameworks that include cargo insurance, risk management, and contractual obligations defined in service level agreements.
A key QA responsibility is conducting regular self-inspections, typically on a quarterly basis. These internal audits assess warehouse operations against both internal standards and external regulatory expectations such as EU GMP or FDA requirements. Self-inspections review documentation accuracy, environmental controls, personnel training, hygiene and pest control, and inventory status management. Their purpose is not to “pass” an audit, but to identify weaknesses early and correct them before they escalate.
| What QA reviews | Why it matters |
|---|---|
| Documentation & records | Proves compliance and data integrity |
| Environmental monitoring | Confirms storage conditions stayed in range |
| Training & SOP adherence | Reduces human error |
| Facility hygiene | Prevents contamination risks |
| Inventory controls | Ensures traceability and recall readiness |
When issues arise, CAPA (Corrective and Preventive Action) provides the structured response. Corrective actions address the immediate problem, while preventive actions eliminate the root cause. For example, a recurring cold spot affecting biologics may require temporary product relocation (corrective) and HVAC or racking adjustments (preventive). Each step is documented and reviewed to confirm effectiveness and ensure continuous improvement.
External inspections by regulators or customer audit teams ultimately test whether the quality system works in practice. Warehouses that run effective self-inspections and CAPA programs are far better prepared for these assessments, even when they occur with little notice.
How to set up a GMP warehouse (EU and US context)
Setting up a GMP warehouse is not a single task – it’s a structured process that builds compliance layer by layer. Whether you’re launching a new facility or upgrading an existing one, the journey typically takes several months and follows a predictable sequence. Getting the order right is what prevents delays, rework, and regulatory findings later.
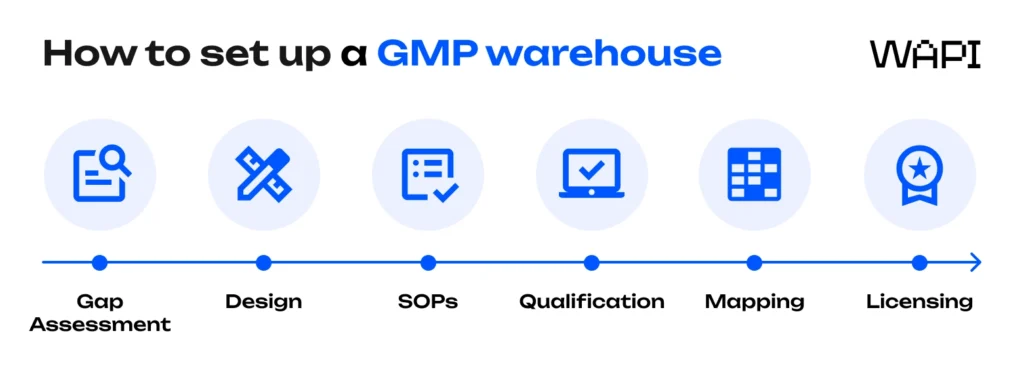
At a high level, GMP warehouse setup follows six core stages, from initial assessment through licensing and inspection. Each step builds on the previous one and supports long-term compliance rather than short-term approval.
Step 1: Gap assessment – understanding where you stand
Every GMP project starts with a gap assessment. This is a structured comparison of your current facility, processes, and documentation against applicable GMP and GDP guidelines.
The goal is not just to identify non-compliance, but to understand why gaps exist and what level of effort is required to close them. For existing warehouses, this step often reveals hidden risks – such as inadequate zoning, insufficient documentation, or training gaps – that are not obvious during day-to-day operations.
What this step typically evaluates:
- Facility layout and storage areas
- Environmental controls and monitoring equipment
- Existing SOPs and documentation
- Staff training and operational practices
Step 2: Facility design or retrofit – building compliance into the layout
Once gaps are clear, attention turns to the physical environment. Facility design (or retrofit) ensures the warehouse can actually support GMP operations, not just on paper, but in practice.
This stage focuses on defining zones for different storage conditions and product statuses, ensuring appropriate segregation of quarantine, released, and rejected goods. Environmental systems must be capable of maintaining validated temperature and humidity ranges, while surfaces, access points, and workflows are designed to reduce contamination and mix-up risks.
Why this matters: design decisions made at this stage directly affect operational efficiency and inspection outcomes for years to come.
Step 3: SOPs and quality system – standardising how work is done
With the physical setup defined, the focus shifts to process control. Developing a Pharmaceutical Quality System establishes how the warehouse operates on a daily basis.
This includes creating standard operating procedures for all critical activities – receiving, storage, picking, dispatch, cleaning, deviation handling, and change control. Risk management processes are embedded to identify and mitigate quality threats before they escalate, ensuring consistency across shifts, teams, and sites.
Rather than reacting to issues, the quality system defines how compliance is maintained continuously.
Step 4: Equipment and system qualification (IQ/OQ/PQ)
Before operations begin, equipment and systems must be formally qualified. This follows the IQ/OQ/PQ model:
- Installation Qualification (IQ): confirms equipment is installed correctly
- Operational Qualification (OQ): verifies systems operate as intended
- Performance Qualification (PQ): demonstrates consistent performance under real conditions
Computer systems used in warehouse operations – such as WMS or monitoring platforms also require validation to ensure data integrity, controlled access, and reliable audit trails.
Step 5: Temperature mapping – proving environmental control
Temperature mapping studies are a mandatory validation step before storing regulated products. These studies assess how temperature behaves across the warehouse under different conditions.
Dozens of data loggers are placed throughout storage areas during both summer and winter, and under empty and fully loaded scenarios. The results identify hot and cold spots and define where specific products can be safely stored.
EU GDP Guidelines explicitly require this step, and inspectors frequently review mapping reports during audits.
Step 6: Licensing and regulatory approval – the final gate
The final stage is regulatory authorisation, which varies by jurisdiction.
EU context:
- Manufacturing and Importation Authorisation (MIA) or
- Wholesale Distribution Authorisation (WDA)
US context:
- State-level distribution licenses
- FDA registration (depending on activities)
Approval typically involves document review followed by an on-site inspection. Timelines can range from a few months to longer, depending on readiness and regulator workload – making early preparation critical.
Typical setup timeline
- Gap assessment: 2–4 weeks
- Design & SOP development: 1–3 months
- Qualification & mapping: 1–2 months
- Licensing & inspection: 2–6 months
Exact timelines vary by facility size, product type, and jurisdiction.
GMP warehouses and ecommerce / outsourced fulfillment
GMP implementation follows a structured path:
- Gap assessment against GMP/GDP guidelines
- Facility design or retrofit
- Quality system and SOP development
- Equipment and system qualification (IQ/OQ/PQ)
- Temperature mapping
- Licensing and regulatory inspection
EU facilities typically require MIA or WDA authorisation, while US facilities need state licensing and FDA registration depending on activities.
Choosing an outsourced fulfillment partner
Many ecommerce brands choose GMP-compliant 3PLs or courier service instead of building their own facilities. This reduces capital investment, helps with supply chain and speeds market entry. Key questions to ask potential 3PL partners include:
- What licenses and certifications does the facility hold, and when was the last regulatory inspection?
- What temperature ranges can you maintain, and how do you monitor and document conditions?
- How do you handle batch tracking, expiry management, and recall execution?
- What training do your warehouse staff receive, and how often is it refreshed?
- Can you provide references from clients with similar products?
Trade-offs between in-house and outsourced approaches merit consideration. Operating your own GMP warehouse provides direct control over every aspect of operations but demands significant investment in facility, equipment maintenance, personnel, and regulatory management. Outsourcing fulfillment to reliable partners like WAPI offers faster time to market and leverages existing expertise but requires robust quality and technical agreements defining responsibilities, specifications, and communication protocols.
A practical example illustrates the outsourcing decision: following Brexit in 2021, a UK supplement brand needed EU-based storage to continue serving European customers without customs complications. Rather than establishing their own facility, they partnered with a GMP-compliant 3PL in the Netherlands that already held appropriate licenses and had experience with dietary supplements. This approach enabled continued EU distribution within weeks rather than the months required for establishing their own operation.
Technology and innovation in GMP warehouses
Digital tools and automation increasingly support both regulatory compliance and operational efficiency in modern GMP facilities. Technology investments that once seemed optional have become essential for maintaining competitive operations while meeting rising regulatory expectations.
Warehouse Management Systems integrated with ERP and ecommerce platforms form the operational backbone of contemporary pharmaceutical warehouses. When validated for GMP use, these systems enforce business rules – preventing picks from quarantine stock, requiring batch and expiry verification, maintaining comprehensive audit trails. Integration with customer order systems enables seamless flow from purchase through fulfillment while maintaining the documentation required for regulatory compliance.
IoT sensors enable real-time temperature and humidity monitoring that goes far beyond periodic manual readings. Networked devices throughout the warehouse transmit data continuously to central platforms where automated alerts notify staff of developing excursions before products are affected. Complete audit trails capture every reading, supporting both internal quality reviews and external inspections.
Electronic Quality Management Systems (eQMS) centralize deviation management, CAPA tracking, training records, and change control in validated platforms. These systems replace paper-based processes that were prone to gaps and delays, enabling faster response to quality issues and more comprehensive documentation.
Advanced facilities explore additional technologies including automated storage and retrieval systems configured to enforce segregation rules and FEFO logic within controlled environments, RFID for pallet and batch identification enabling faster and more accurate stock counts, and blockchain-style traceability for high-value products or controlled substances requiring enhanced chain-of-custody documentation.
Regulatory expectations for computerized systems continue to evolve. Data integrity requirements mandate that electronic records be attributable, legible, contemporaneous, original, and accurate. User access controls must limit system functions to authorized personnel. Audit trails must capture all changes with timestamps and user identification. Compliance with frameworks like 21 CFR Part 11 and EU Annex 11 requires ongoing attention as systems are implemented and maintained.
Conclusion
A GMP warehouse is defined not by paperwork, but by how well systems, people, and infrastructure protect pharmaceutical products every day. By embedding good manufacturing practice, gmp regulations, and continuous improvement into warehouse operations, compliant pharmaceutical warehouses ensure safe storage and distribution in regulated markets.
For growing brands, GMP warehousing is no longer optional. Whether outsourcing or scaling internally, aligning early with GMP standards is the most reliable way to maintain compliance, protect customers, and scale with confidence.
FAQs on a GMP warehouse operations
Understanding the GMP warehouse often raises additional questions about how these facilities operate in practice. The following questions address common inquiries, starting with GMP regulations and ending with distribution process.
Is a GMP warehouse the same as a GDP warehouse?
GMP (Good Manufacturing Practice) and GDP (Good Distribution Practice) overlap significantly but address different primary activities. GMP focuses on manufacturing, processing, and holding of products, while GDP specifically addresses wholesale distribution and transport. A warehouse attached to a manufacturing site typically operates under GMP, while a distribution center serving wholesalers operates under GDP. In practice, many facilities implement both sets of requirements, and the terms are sometimes used interchangeably when describing storage operations for pharmaceutical products.
Do all pharmaceutical products need to be stored in a GMP-compliant warehouse?
Regulatory requirements generally mandate that medicinal products be stored under conditions that maintain their quality and safety throughout the distribution chain. This effectively requires GMP or GDP-compliant storage for prescription drugs, biologics, and most OTC medicines. Some lower-risk products like certain medical devices or cosmetics may have less stringent requirements depending on local regulations. Risk-based approaches allow companies to calibrate controls to product characteristics, but the fundamental expectation of appropriate storage conditions applies broadly.
How long does it take to get a GMP warehouse approved?
Timeline varies significantly based on facility readiness, regulatory workload, and jurisdiction. In the EU, applicants should expect several months from initial application submission through inspection and final authorisation. The process includes document review, scheduling of inspection, on-site assessment, and resolution of any findings. Companies planning new facilities should build regulatory approval time into project schedules, as operations cannot begin until authorisation is granted. Pre-submission consultations with competent authorities can help identify potential issues early.
What is the difference between GMP certification and ISO 9001 for warehouses?
GMP represents regulatory requirements specific to pharmaceutical products and related goods, enforced by government bodies like the FDA or European Medicines Agency. ISO 9001 is a voluntary quality management standard applicable across industries. A warehouse can be ISO 9001 certified without being GMP compliant, and vice versa. Pharmaceutical companies generally require GMP compliance as a baseline because it addresses product-specific risks and satisfies legal obligations. ISO 9001 may complement but cannot substitute for GMP compliance when storing regulated products.
Can a 3PL warehouse be GMP-certified if it serves multiple industries?
Yes, many 3PL providers operate GMP-compliant areas within larger facilities that also serve non-regulated industries. The key requirement is adequate separation – physical, procedural, and system-based – between GMP and non-GMP operations. Dedicated storage zones, trained personnel assigned to pharmaceutical operations, and validated systems for regulated products enable this multi-use approach. Clients should verify through qualification audits that the 3PL maintains appropriate controls and that their products won’t be compromised by proximity to non-compliant operations.
What documentation must a GMP warehouse provide during a customer or regulator audit?
Auditors typically request evidence across the full quality system: facility qualification records including temperature mapping studies, equipment calibration certificates, cleaning and pest control records, environmental monitoring data, training records for all personnel handling products, deviation and CAPA logs, inventory records with batch traceability, and distribution records showing chain of custody. The specific documentation depends on audit scope, but warehouses should maintain records readily accessible and organized for review on short notice.

 Community
Community
